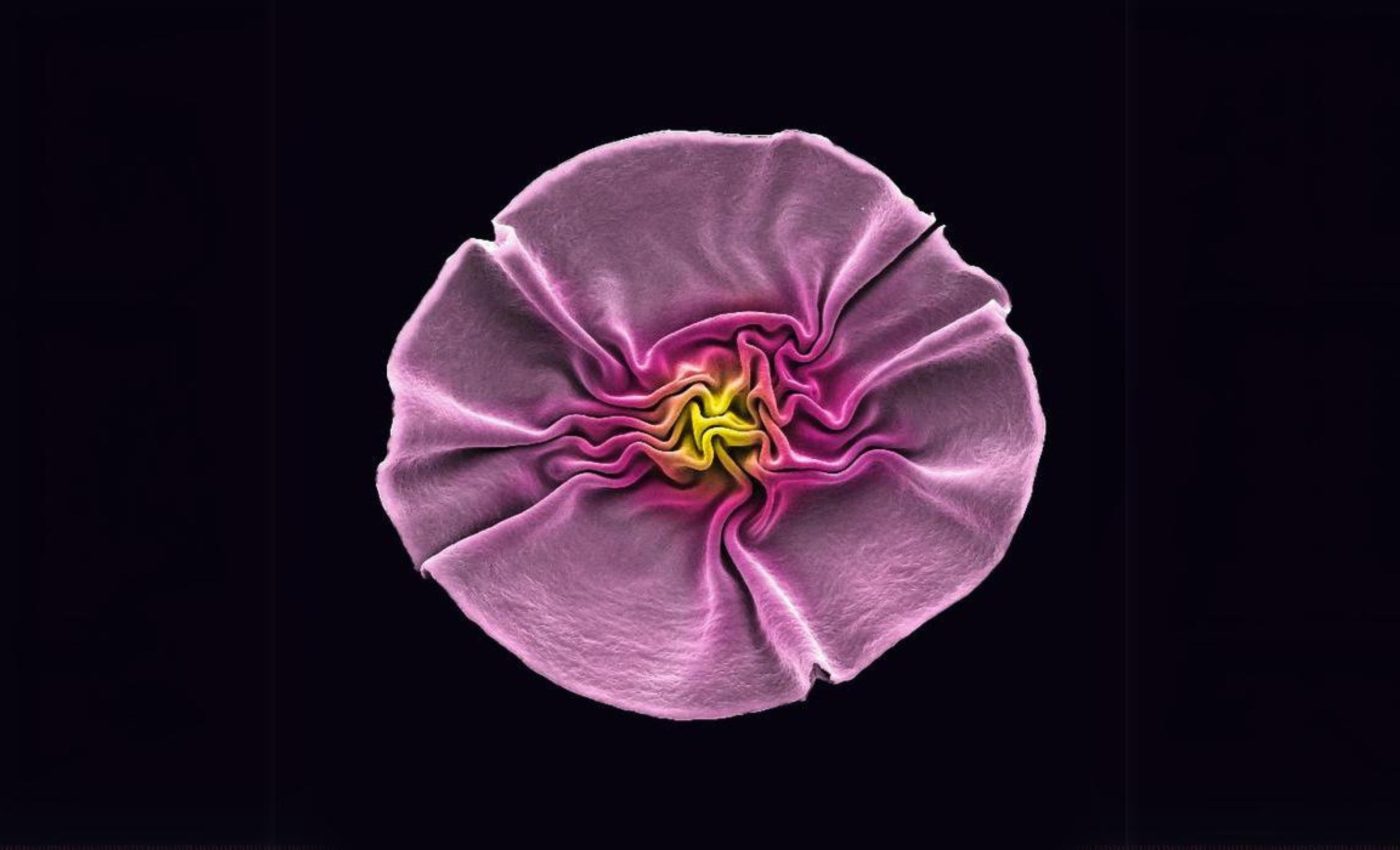
Tiny viruses link up and bloom into amazing sunflower structures
In the world of scientific discovery, some of the most exciting breakthroughs happen by accident. Researchers often begin with a clear plan, only to find that nature has its own surprises, such as viruses that look like a sunflower.
This was exactly the case for a group of scientists, who, while working with bacteriophages – viruses that target and eat bacteria – stumbled upon something unexpected. What they discovered were tiny, sunflower-like viruses that could transform the way we approach disease detection and treatment.
Viruses that look like a sunflower?
It seems that even the tiniest of lifeforms enjoy getting a little creative sometimes. The McMaster team was left stunned at their lab slides when they observed the bacteriophages, often referred to as phages, forming into 3D configurations closely resembling sunflowers.
But these tiny “flowers” were not your average garden variety. They were just two-tenths of a millimeter across.
Isn’t it surprising how sometimes, momentous discoveries can pop up in the most unexpected places?
Unexpected formation of phages
What sparked this peculiar metamorphosis? The answer lies in the process the researchers had used to prepare the phages for viewing.
In order to keep the viruses alive, the researchers decided on the path less traveled by using high-pressure carbon dioxide instead of the standard and more lethal heat or solvent-based methods.
And the result? An unexpected yet promising microstructure that they had been aiming to synthesize for years.
Study lead author Lei Tian, who led the research during his time as a PhD student and subsequently, a postdoctoral research fellow at McMaster, said his initial intention was only to preserve the virus structure. But nature, as always, had other plans.
Significance of the sunflower formations
What makes this haphazard structure so important? Well, it turns out to be 100 times more capable than unlinked phages at locating their bacterial targets.
This means that these sunflower-like formations could open up a world of opportunities in detecting and combating numerous diseases. And the best part – all of this is achieved via natural materials and processes.
Incredible insight on sunflower-like viruses
“It was an accidental discovery,” said study co-author and mechanical engineer Tohid Didar. “When we took them out of the high-pressure chamber and saw these beautiful flowers, it completely blew our minds.”
“It took us two years to discover how and why this happened and opened the door to being able to create similar structures with other protein-based materials.”
Zeinab Hosseinidoust, senior author of the study, has been exploring the potential of phages in her lab for years now.
The team has made significant strides, such as prompting the viruses to interlink, forming a microscopic living fabric, and even developing a gel visible to the naked eye.
In the past, it hasn’t been possible to give this material any form of depth or shape. But with the arrival of the sunflower-like structures of viruses with all their wrinkles, peaks, and crevices, it seems that barrier has been broken down.
Nature’s masterpiece: Sunflower-like viruses
“This is really about building with nature,” said Hosseinidoust. “This kind of beautiful, wrinkled structure is ubiquitous in nature.”
“The mechanical, optical and biological properties of this kind of structure have inspired engineers over decades to build these kinds of structures artificially, in the hope of getting the same kind of properties out of them.”
The discovery has opened doors to shaping phages in a variety of ways, enhancing their efficiency and creating opportunities for innovative biological applications.
For instance, the team was able to blend these flower-like structures with DNAzymes, enabling them to detect low concentrations of Legionella bacteria in water from commercial cooling towers.
Endless source of inspiration
As antibiotic resistance continues to be a major concern, a rekindled interest in bacteriophages as a treatment for various infections has arisen.
With their ability to specifically target certain bacteria while leaving others unharmed, the potential for these tiny viruses holds a lot more promise than we might have believed.
“Nature is so powerful and so intelligent. As engineers, it’s our job to learn how it works, so we can harness processes like this and put them to use,” said Hosseinidoust.
The study is published in the journal Advanced Functional Materials.
Image Credit: McMaster University
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













