Tiny animals steal genes from bacteria to produce antibiotics
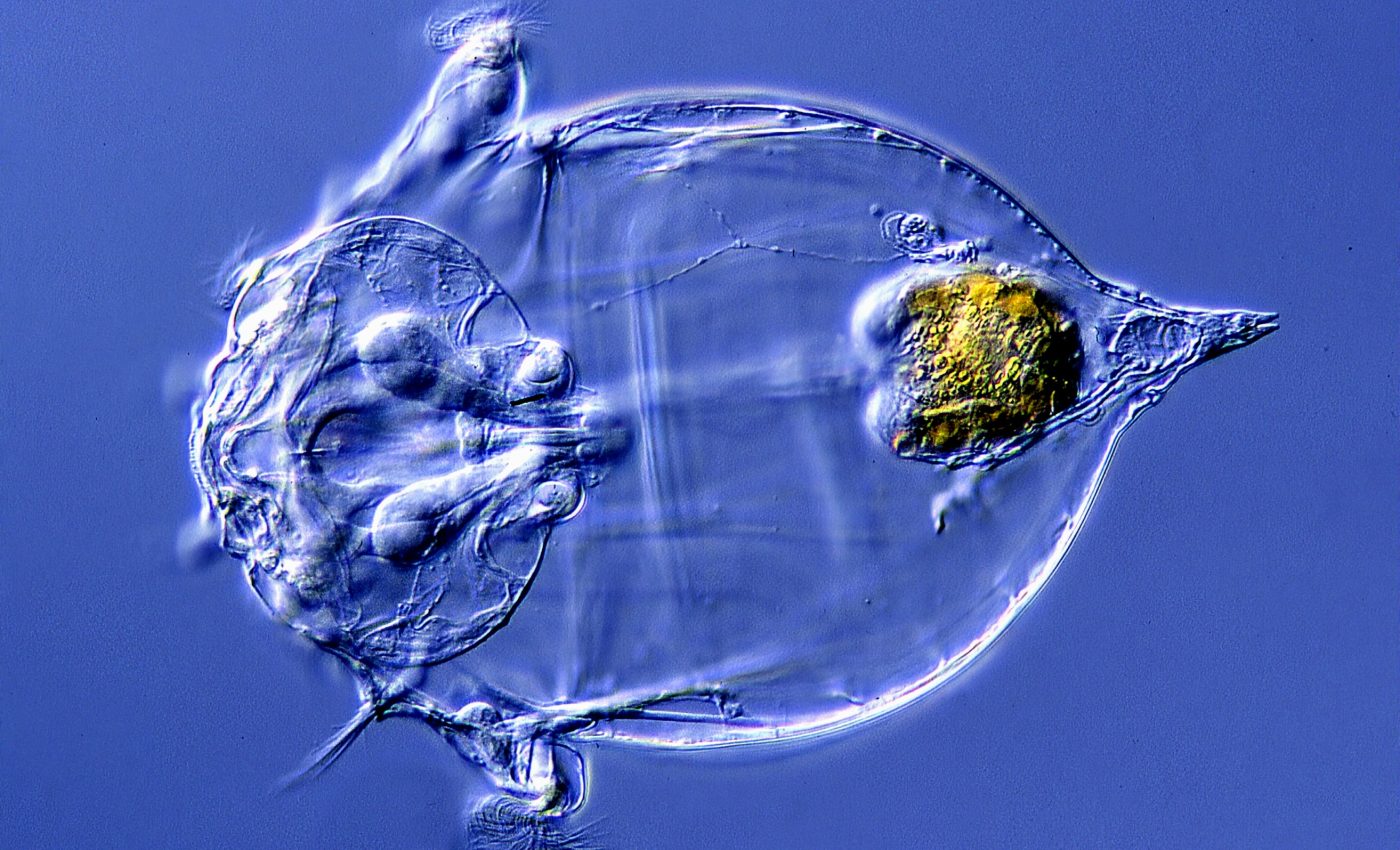
A microscopic marvel of our planet, the bdelloid rotifer, is an aquatic critter not much larger than the width of a hair. Fascinatingly, these tiny creatures can arm themselves against infections using “stolen” antibiotics from bacteria.
This intriguing discovery was made by researchers at the University of Oxford, University of Stirling, and the Marine Biological Laboratory, Woods Hole.
Rotifers: Shaping the future of antibiotics
No bigger than a quarter of a millimeter, these bdelloid rotifers are resourceful survivors. When exposed to a potentially fatal fungal infection, they activate hundreds of bacterial and microbial genes they possess.
These genes churn out a diverse arsenal of antimicrobial weapons, including antibiotics, to fend off the invading pathogens.
“When we translated the DNA code to see what the stolen genes were doing, we had a surprise. The main genes were instructions for chemicals that we didn’t think animals could make – they looked like recipes for antibiotics,” noted Chris Wilson, from the University of Oxford, a co-author involved in this study.
Magic of evolution
Research has shown that bdelloid rotifers have been absorbing DNA from their environment for millions of years. However, this recent study is the first to document these creatures utilizing the absorbed genes as an effective defense mechanism against diseases.
Borrowing genes from other organisms is not a common animal trait. This unusual ability of rotifers has captivated David Mark Welch, senior scientist and director of the Josephine Bay Paul Center at the Marine Biological Laboratory.
“These complex genes were acquired from bacteria but have undergone evolution in rotifers,” said Welch.
Could rotifers be the key to better antibiotics?
Exploring the rotifers’ unique genetic abilities might not only answer fundamental evolutionary questions, but also aid in the development of new antibacterial and antifungal drugs.
Today’s pressing concern of antibiotic resistance could potentially find a solution in these diminutive heroes.
“The recipes the rotifers are using look different from known genes in microbes… it might suggest ideas for future medicines,” said Reuben Nowell, from the University of Stirling.
Intriguingly, the bdelloid rotifers produce non-ribosomal peptides, small amino acid-based molecules.
Irina Arkhipova, senior scientist at the Marine Biological Laboratory, said the crucial next step would involve identifying these non-ribosomally synthesized peptides produced by bdelloid rotifers, and the conditions that prompt their synthesis.
Finding safer drugs: A possibility?
One of the major hurdles of drug development is the toxic side effects common in antibiotics produced by bacteria and fungi.
If bdelloid rotifers are indeed producing similar chemicals in their cells, they might be less toxic and safer for use in other animals, potentially paving the path to less toxic medicines for humans.
Peculiarities of the bdelloid rotifers
There’s a peculiar characteristic of bdelloid rotifers that deeply interests researchers – their asexual reproduction.
Unlike most animals, rotifers reproduce through parthenogenesis, where the offspring is an exact genetic clone of the mother, without the need for sexual reproduction.
“If rotifers don’t find a way to change their genes, they could go extinct. This might help explain why these rotifers have borrowed so many genes from other places, especially anything that helps them cope with infections,” said Tim Barraclough, a study co-author from the University of Oxford.
Biodiversity and bdelloid rotifers
Bdelloid rotifers not only hold potential for medical advancements but also play a crucial role in preserving aquatic ecosystems.
These resilient organisms contribute to nutrient cycling by breaking down organic matter, which in turn supports the growth of aquatic plants and microorganisms. Their presence helps maintain the balance of micro-ecosystems, ensuring that water bodies remain healthy and productive.
Understanding the ecological roles and conservation needs of bdelloid rotifers can help in developing strategies to protect diverse aquatic habitats, promoting the health of larger environmental systems.
Rotifers vs. antibiotic resistance
The groundbreaking discoveries concerning bdelloid rotifers have opened up numerous avenues for further research to tackle antibiotic resistance.
Scientists are particularly interested in sequencing the rotifer genome in more detail to uncover additional hidden genes that may offer further insights into their extraordinary capabilities.
Moreover, investigating the mechanisms by which rotifers incorporate foreign DNA into their genomes could yield significant advancements in genetic engineering and biotechnology.
Future of rotifers and antibiotics
With a wealth of genes borrowed from bacteria, plants, and other forms of life, the bdelloid rotifers present an exciting avenue for scientific exploration.
The findings from this research shed light on the fascinating nature of gene movement between different forms of life, offering valuable insights for biological research and medical advancements.
The tale of these tiny survivors, armed with borrowed genes and antibiotic recipes, is a reminder that sometimes nature’s smallest creatures might just hold an answer to some of our biggest challenges.
The study is published in the journal Nature Communications.
—–
Like what you read? Subscribe to our newsletter for engaging articles, exclusive content, and the latest updates.
Check us out on EarthSnap, a free app brought to you by Eric Ralls and Earth.com.
—–













